REGSIGN (REGulatorySIGNature)
To decode Treg signatures in cancer in order to:
- Develop a Treg-based “liquid” biopsy to predict response to immunotherapy & autoimmune side effects.
- Generate innovative tools for identification & validation of therapeutic targets.
RegSign: An innovative, high-risk project with feasible outcomes
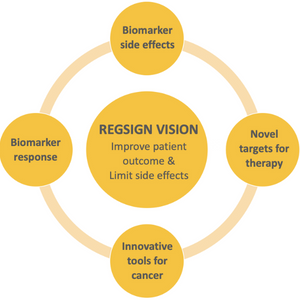
RegSign Overview
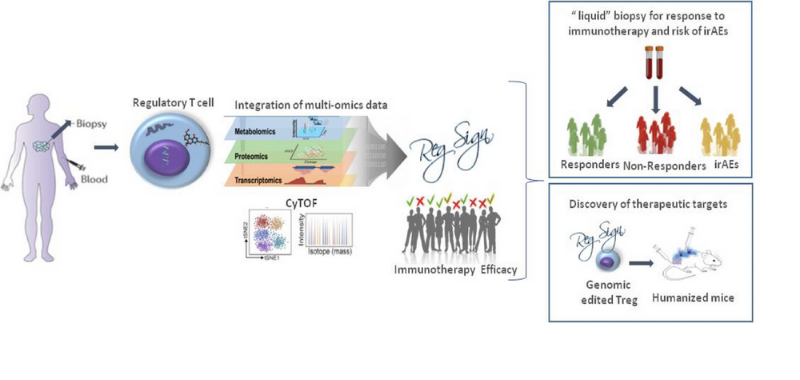
Project Overview
Immunotherapy has revolutionized cancer treatment. However, more than half of treated patients do not show beneficial clinical outcomes whereas responses are often accompanied by severe
autoimmune side effects. Predictive biomarkers of clinical response and novel approaches to master anti-tumor immunity while keeping in check autoimmune responses are very important challenges
in oncology that can open the path to effective treatment of cancer.
It is the ambition of REGSIGN to answer fundamental research questions and provide diagnostic tools to improve the quality of care for cancer patients. RegSign through multi-parametric
single-cell analysis will set the stage for exploring novel concepts in cancer, such as the role of circulating regulatory T cells, that represent one of the main obstacles for effective
immunotherapies, to serve as a “liquid” biopsy for predicting response to treatment and development of autoimmune manifestations. Furthermore, this project will generate innovative animal
models, that will allow the development and testing of new immunotherapeutic targets and design of personalized therapy. Finally, RegSign will tackle with a pioneering concept of
modulating Treg metabolism to adjuvant immune system to fight cancer and simultaneously keep in track hazardous attacks on self tissue, that will generate breakthroughs in management of
immunotherapy-induced autoimmune side effects.